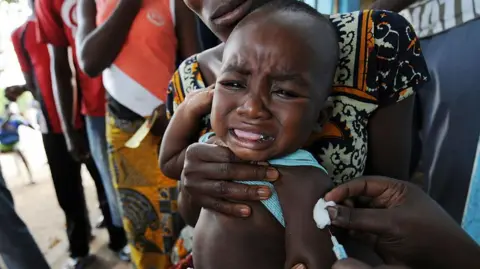
WHO Condemns US-Funded Vaccine Trial in Guinea-Bissau as Unethical
A now-halted plan to run a hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau has been criticized by the World Health Organization as unethical. The US-funded study sought to give one set of babies the vaccine at birth while another would have had the shot delayed until six weeks of age.
The WHO expressed significant concerns, stating that the birth-dose vaccine is an essential public health intervention with a proven track record. The trial was led by the US health department, under Robert F Kennedy Jr., who raised questions regarding the broader health effects of the vaccine.
The WHO highlighted the scientific and ethical concerns regarding the study, asserting that the hepatitis B birth-dose vaccine has been employed successfully in over 115 countries for three decades. They stressed that depriving some newborns of this life-saving intervention could lead to potentially irreversible harm.
With a sizeable portion of Guinea-Bissau's population estimated to carry hepatitis B, vaccination at birth can prevent mother-to-baby transmission in 70-95% of cases, according to the WHO. Moreover, the organization pointed out that ethical trials allowing placebos are only applicable when no proven treatment exists, a point that does not apply here.
In Guinea-Bissau, the hepatitis B vaccine is currently administered at six weeks, but authorities are planning to introduce the birth dose nationwide by 2028. The highly criticized trial was to include 14,000 babies and was led by Danish researchers.
Public outrage, amplified by ethics concerns, led the Guinea-Bissau government to suspend the project last month. Critics questioned the appropriateness of conducting such trials on infants in an African context, with the former health minister, Magda Robalo, stating, Guinea-Bissauans are not guinea pigs.
Recent decisions by a US advisory panel to stop recommending immediate hepatitis B vaccination for all newborns added to the controversy, as did the fact that the panel was appointed by Secretary Kennedy after he dismissed previous committee members who generally supported vaccinations.
More than 12% of Guinea-Bissau's adult population lives with chronic hepatitis B, with estimates suggesting the rate could be as high as one in five. The WHO continues to advocate for newborns receiving the hepatitis B vaccine within 24 hours of birth, emphasizing the potential for chronic carriers among newborns infected at birth.