The first malaria treatment tailored for infants has received official approval, marking a significant milestone in the fight against malaria. Developed by pharmaceutical leader Novartis, this pioneering medication is set to be introduced across African countries soon, where malaria's impact has been devastating, particularly for children.
The year 2023 recorded an alarming 597,000 malaria-related deaths, predominantly in Africa, where children under five accounted for approximately 75% of these tragedies. Prior to this approval, infants had to rely on medications formulated for older children, raising significant risks of overdose due to their different physiology and developing organ systems.
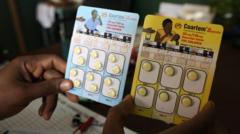
The newly approved drug, referred to as Coartem Baby (or Riamet Baby in certain regions), has been brought to fruition through the collaboration of Novartis and the Medicines for Malaria Venture, a non-profit organization aimed at tackling this critical health issue. The Swiss authorities' approval signals a turning point in the healthcare landscape for the youngest and most vulnerable populations.
Chief executive of Novartis, Vas Narasimhan, expressed his commitment to combating malaria through continued innovation. He stated, "This is a pivotal moment in our relentless pursuit to provide medical solutions where they are most needed." Along with a commitment to distribute the medication on a not-for-profit basis, this initiative aims to make vital health resources more equitable.
The approval has been welcomed by health experts who highlight the need for specialized treatments capable of addressing the unique challenges faced by infants suffering from malaria. Dr. Marvelle Brown from the University of Hertfordshire points out the urgency of this issue in sub-Saharan Africa, where the high death rate among young children from malaria is a critical public health concern.
The introduction of Coartem Baby is considered a beacon of hope, with calls for a systematic approach to tackle malaria-related mortality and improve healthcare access for all socio-economic groups. As the rollout approaches, many anticipate a positive impact on public health strategies and outcomes in the regions most affected by this disease.