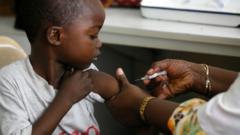
The first malaria treatment specifically designed for newborns and very young children has received approval for widespread use, paving the way for its introduction in African nations within weeks. Previously, infants were treated with medications formulated for older children, leading to the risk of overdose due to differing dosages, especially critical given their developing liver functions and body systems.
Malaria is a significant health concern, with around 597,000 deaths linked to the disease in 2023, predominantly affecting young children in Africa. The vulnerability of this demographic underscores the urgent need for suitable treatments, as three-quarters of malaria-related deaths occur in children under five years old.
The new drug, known as Coartem Baby, was developed by Novartis in collaboration with the Medicines for Malaria Venture (MMV) and is expected to be distributed without profit primarily to the hardest-hit regions. Novartis CEO, Vas Narasimhan, emphasized the importance of this development, stating that it represents a commitment to combatting malaria through innovative medical solutions.
The drug's formulation caters specifically to those weighing less than 4.5kg (10lb), addressing a critical treatment gap that has existed for over three decades. Martin Fitchet, CEO of MMV, highlighted the necessity of this targeted treatment, labeling malaria one of the deadliest diseases, primarily affecting children. Fitchet emphasized that with the right resources, real progress could be made toward malaria elimination.
Experts in child health, like Dr. Marvelle Brown from the University of Hertfordshire, herald this approval as a major breakthrough in saving the lives of infants particularly in areas heavily impacted by malaria. With an alarming percentage of deaths in malarial infections occurring in those under five, this targeted treatment is a vital step toward addressing this health crisis and promoting equitable access to healthcare solutions.
Malaria is a significant health concern, with around 597,000 deaths linked to the disease in 2023, predominantly affecting young children in Africa. The vulnerability of this demographic underscores the urgent need for suitable treatments, as three-quarters of malaria-related deaths occur in children under five years old.
The new drug, known as Coartem Baby, was developed by Novartis in collaboration with the Medicines for Malaria Venture (MMV) and is expected to be distributed without profit primarily to the hardest-hit regions. Novartis CEO, Vas Narasimhan, emphasized the importance of this development, stating that it represents a commitment to combatting malaria through innovative medical solutions.
The drug's formulation caters specifically to those weighing less than 4.5kg (10lb), addressing a critical treatment gap that has existed for over three decades. Martin Fitchet, CEO of MMV, highlighted the necessity of this targeted treatment, labeling malaria one of the deadliest diseases, primarily affecting children. Fitchet emphasized that with the right resources, real progress could be made toward malaria elimination.
Experts in child health, like Dr. Marvelle Brown from the University of Hertfordshire, herald this approval as a major breakthrough in saving the lives of infants particularly in areas heavily impacted by malaria. With an alarming percentage of deaths in malarial infections occurring in those under five, this targeted treatment is a vital step toward addressing this health crisis and promoting equitable access to healthcare solutions.