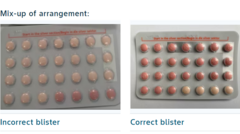
Bayer Ltd, the manufacturer of Yaz Plus, has initiated the recall of a specific batch of the contraceptive pill due to a significant packaging error. The South African Health Products Regulatory Agency reported that some blister packs within this batch mistakenly contained 24 inactive pills instead of the standard 24 active hormone-containing pills. This mix-up raises concerns about unintended pregnancies for women who may have unknowingly ingested inactive tablets, presuming they were receiving effective contraception.
The affected batch, identified as WEW96J and set to expire in March 2026, has prompted Bayer Ltd to urge users to stop taking these pills immediately and consult their healthcare providers. The company's recall notice emphasized the importance of not using any tablets from the erroneous packs until proper consultation has occurred, underscoring that these might not afford the expected contraceptive protection.
While Bayer reported that only a limited number of packs from this one specific batch are in circulation, they recommend that anyone in possession of the affected product return it to pharmacies in exchange for a replacement or refund. The company has communicated with healthcare professionals, hospitals, and suppliers to advise them of the recall and request the return of any affected items.
Bayer Ltd has stated that the root cause of the mix-up has been identified and that corrective actions have been implemented to prevent future occurrences. To assist those with questions regarding this situation, the company has also established a dedicated helpline.
The affected batch, identified as WEW96J and set to expire in March 2026, has prompted Bayer Ltd to urge users to stop taking these pills immediately and consult their healthcare providers. The company's recall notice emphasized the importance of not using any tablets from the erroneous packs until proper consultation has occurred, underscoring that these might not afford the expected contraceptive protection.
While Bayer reported that only a limited number of packs from this one specific batch are in circulation, they recommend that anyone in possession of the affected product return it to pharmacies in exchange for a replacement or refund. The company has communicated with healthcare professionals, hospitals, and suppliers to advise them of the recall and request the return of any affected items.
Bayer Ltd has stated that the root cause of the mix-up has been identified and that corrective actions have been implemented to prevent future occurrences. To assist those with questions regarding this situation, the company has also established a dedicated helpline.